COMPOUND PROPERTY CALCULATION & REMOVAL OF UNDESIRABLES
FILTER
FILTER is a very fast molecular filtering and selection application. It uses a combination of physical property calculations and functional group knowledge to remove undesirable compounds before they enter experimental or virtual screening.
Undesirable properties may include: toxic functionalities, a high likelihood of binding covalently with the target protein, interfering with the experimental assay, and/or a low probability of oral bioavailability.
Eliminating unwanted molecules before the use of modeling applications will, in turn, substantially increase the positive predictive value of these tools, and significantly reduce their processing time.
For more detailed information on FILTER, please click below:
Documentation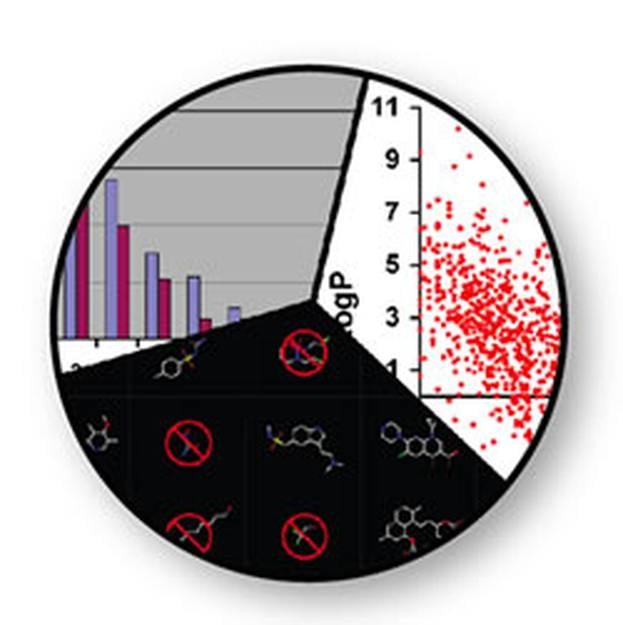
Removing undesirable compounds early makes the downstream processes significantly more efficient; a simple concept that is too often ignored.
Features
- Uses predefined filter files - text files that can be easily customized
- Filters based upon calculated properties such as: MW, XlogP [1], XlogS, PSA [2], hydrogen bond donor and acceptor count, rotatable bonds, ring size and number, etc.
- Removes or retains molecules based upon pre-defined or user-defined substructures
- Assigns graph-based protonation state for consistency and speed
- Offers ADME filters such as Lipinski [3], Egan [4], Veber [5] and Martin [6]
- Removes compounds with impossible bonding and inappropriate elements
- Generates tab-separated files suitable for import into spreadsheets
- Processes 400 mol/sec
References
- A New Atom-Additive Method for Calculating Partition Coefficients Wang, R., Ying, F., and Lai, L., J. Chem. Inf. Comput. Sci., 1997, 37, 615.
- Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties Ertl, P., Rohde, B., and Selzer, P., J. Med. Chem., 2000, 43, 3714.
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings Lipinski, C., et al., Adv. Drug Deliv. Rev., 1997, 23, 3.
- Prediction of drug absorption using multivariate statistics Egan, W.J., Merz, K.M., Baldwin, J.J., J. Med. Chem., 2000, 43, 3867.
- Molecular Properties That Influence the Oral Bioavailability of Drug Candidates Veber, D.F., Johnson, S.R., Cheng, H.Y., Smith, B.R., Ward, K.W., Kipple, K.D., J. Med. Chem., 2002, 45, 2615.
- A bioavailability score Martin, Y.C., J. Med. Chem., 2005, 48, 3164.
ROCS X: AI-Enabled Molecular Search Unlocks Trillions
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch
Science Brief: Binding Free Energy Redefined: Accurate, Fast, Affordable
CUP XXV - Santa Fe March 10-12, 2026
Webinar: Novel Hits from Beyond the Known: ROCS X
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch
Science Brief: Binding Free Energy Redefined: Accurate, Fast, Affordable
CUP XXV - Santa Fe March 10-12, 2026
Webinar: Novel Hits from Beyond the Known: ROCS X
Resources
Glimpse the Future through News, Events, Webinars and more
News
ROCS X: AI-Enabled Molecular Search Unlocks Trillions
BOSTON, OpenEye miniCUP—Sept. 25, 2025—Cadence Molecular Sciences (OpenEye), a business unit of Cadence (Nasdaq: CDNS), announced today at miniCUP Boston, the launch of ROCS X, an AI-enabled virtual screening solution that allows scientists to conduct 3D searches of trillions of drug-like molecules.
Read now
Webinar
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch We are continuing our 2025 miniWEBINAR series with our November session led by Chris Neale, Ph.D., who leads the molecular binding affinity solution group at OpenEye, Cadence Molecular Sciences. His expertise and interests include method development for structure-based drug design, cellular signal transduction, and uncertainty quantification. Chris is also an editorial board member at the Biophysical Journal.
Read now


