- Solutions
- Product
- Orion® Molecular Design Platform
- Formulations Suite
Orion Formulations Suite
Find Promising Formulation Candidates with Crystal Structure Prediction
The Orion® Formulations Suite includes robust crystal structure prediction (CSP) workflows and intrinsic solubility calculations that lets researchers uncover promising candidates for formulation early in the drug development process.
Benefits of Orion Formulation Suite:
-
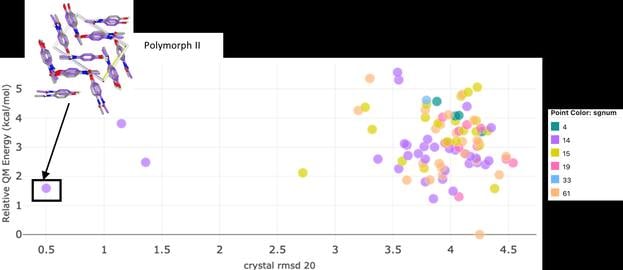
Walk through a series of steps that use crystal-specific force fields and Quantum Mechanics (QM)-energy models. Eliminate high-energy packings that are more likely to be unstable and identify tightly packed, low-energy crystal structures that are more likely to be stable and suitable candidates for formulation.
-
Reduce the likelihood of discovering polymorphs during later stages of development, or even after approval, thereby minimizing the chances of unexpected safety concerns, drug recalls, and financial losses. The computational CSP method in Orion lets researchers explore the polymorph landscape more thoroughly than possible with time-consuming and costly experimental methods.
-
Break free from hardware and compute-time constraints by leveraging the massive scaling made possible when running Orion on Amazon Web Services (AWS). From geometry optimization to entropy calculation, Orion employs novel parallelization methods that return results quickly and accurately, even for large datasets. What used to take days now takes only hours.

White Paper: How to Speed Up Your Drug Development with Crystal Structure Prediction
Download White PaperThe Crystal Structure Prediction Workflow
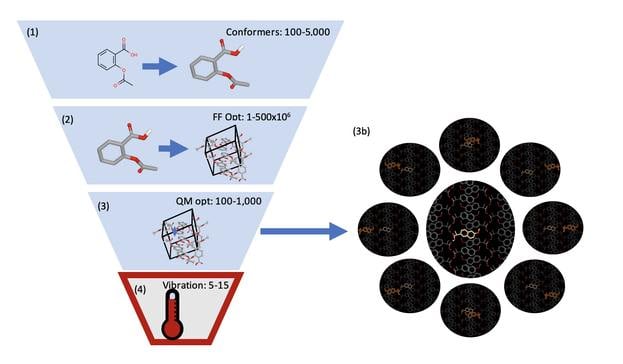
The Orion CSP method was validated in collaboration with researchers at GlaxoSmithKline using several drug-like molecules from pharmaceutical settings. It includes four key stages.
-
Predict the potential 3D conformations of a molecule that might be observed in experimental conditions. This step uses a QM energy model to perform automated torsion scanning of all rotatable bonds and lets users keep only the tautomers that are predicted to be accessible in a defined energy range.
-
Eliminate high-energy packings that are more likely to be unstable and identify tightly packed, low-energy crystal structures that are more likely to be stable and suitable candidates for formulation. This step leverages OpenEye’s proprietary multipole-based intermolecular energy force field (IEFF) for fast and accurate energy calculations.
-
Narrow down the list of crystal candidates by examining differences between long-range, mid-range and short-range interactions and how important they are to a crystal’s stability. This step uses a unique, highly parallelizable dimer-expansion approach that allows for QM optimization of crystal packings in unprecedented time.
-
Predict how temperature will impact the stability of promising crystal structures. OpenEye’s dimer-expansion approach makes crystal entropy calculations highly parallelizable which means estimating the stability of crystals at room temperature can be done prospectively on structures.
Additional Resources:
- Recorded Presentation on Multi-component CSP: Update on multi-component CSP (Crystal Structure Prediction) at OpenEye, Presented by Caitlin Bannan, PhD, Scientific Software Developer at OpenEye Scientific (July 2021)
- Recorded Presentation on Single-component CSP: Ab initio prediction of small-molecule crystal structures: A blind challenge study, Presented by Hari Muddana, Senior Scientific Software Developer at OpenEye Scientific (October 2020)
Prefer to have Crystal structure prediction as a service? CONTACT US
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch
Science Brief: Binding Free Energy Redefined: Accurate, Fast, Affordable
CUP XXV - Santa Fe March 10-12, 2026
Webinar: Novel Hits from Beyond the Known: ROCS X
Resources
Glimpse the Future through News, Events, Webinars and more
News
ROCS X: AI-Enabled Molecular Search Unlocks Trillions
Webinar
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch


