MolProp TK
The MolProp TK provides a customizable framework for molecular property calculation geared towards enabling rapid database filtering. Filtering attempts to eliminate inappropriate or undesirable compounds from a large set before beginning to use them in modelling studies.
The goal is to remove all of the compounds that should not be suggested to a medicinal chemist as a potential hit. This exercise is obviously case dependent, depending on ease of the assay, intended target, personal bias of the modeller & medicinal chemist, strengths of the company, etc., which makes this problem one that is highly amenable to a toolkit solution.
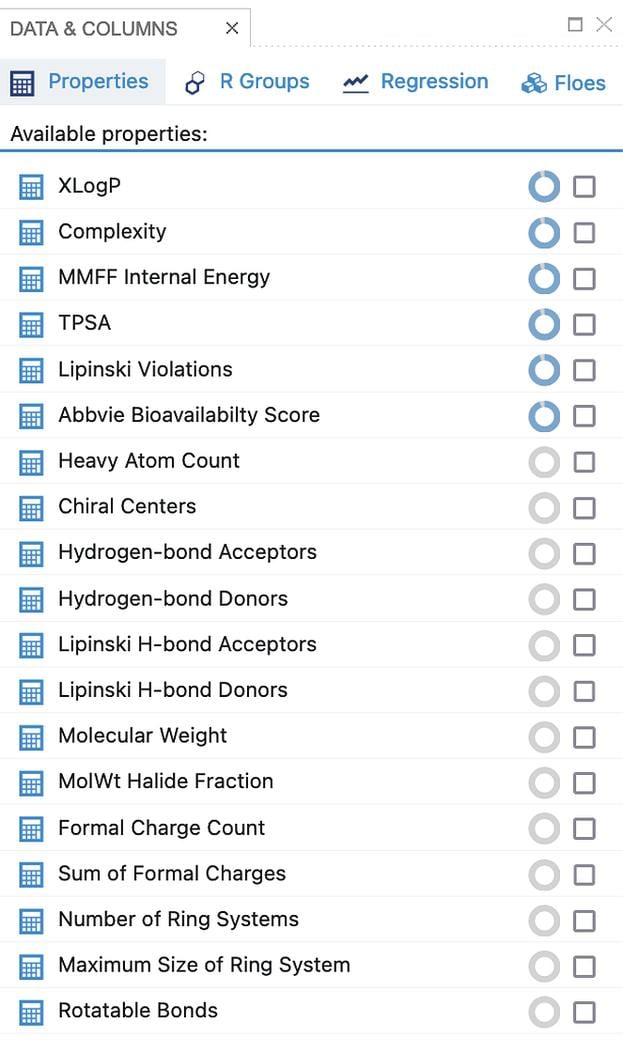
The criteria for passing or failing a given molecule fall into three categories:
Physical properties
- Molecular weight
- Topological polar surface area (TPSA)
- logP
- Bioavailability
Atomic and functional group content
- Absolute and relative content of heteroatoms
- Limits on a very wide variety of functional groups
Molecular graph topology
- Number and size of ring systems
- Flexibility of the molecule
- Size and shape of non-ring chains
Beyond the standard molecular properties available from OEChem TK such as molecular weight and atom type counts, MolProp TK calculates XlogP [1], XlogS, and PSA [2]. There is also a variety of ADME filters available such as Lipinski [3], Egan [4], Veber [5] and Martin [6].
In addition to calculating properties and filtering on those properties, MolProp TK provides a variety of preprocessing tools for metal and salt removal, pKa normalization, normalization, reagent selection and type checking.
For more detailed information on MolProp TK, check out the link below:
DocumentationModeling
The Modeling suite of toolkits provides the core functionality underlying OpenEye's defining principle that shape & electrostatics are the two fundamental descriptors determining intermolecular interactions. Many of the toolkits in the Modeling suite are directly associated with specific OpenEye applications and can, therefore, be used to create new or extend existing functionality associated with those applications.
- OEChem TK Core chemistry handling and representation as well as molecule file I/O
- FastROCS™ TK Real-time shape similarity for virtual screening, lead hopping & shape clustering
- OEDocking TK Molecular docking and scoring
- Omega TK Conformer generation
- Shape TK 3D shape description, optimization, and overlap
- SiteHopper TK Rapid comparison of protein binding sites
- Spicoli TK Surface generation, manipulation, and interrogation
- Spruce TK Protein preparation and modeling
- Szybki TK Force field based focused optimization and entropy estimations
- OEFF TK Force fields and general optimization tools
- Szmap TK Understanding water interactions in a binding site
- Zap TK Calculate Poisson-Boltzmann electrostatic potentials
- Bioisostere TK 3D fragment similarity, lead optimization, and analog generation
- Eon TK Molecular shape and electrostatic overlap
Cheminformatics
The Cheminformatics suite of toolkits provides the core foundation upon which all the OpenEye applications and remaining toolkits are built.
- OEChem TK Core chemistry handling and representation as well as molecule file I/O
- OEDepict TK 2D Molecule rendering and depiction
- Grapheme™ TK Advanced molecule rendering and report generation
- GraphSim TK 2D molecular similarity (e.g. fingerprints)
- Lexichem™ TK Name-to-structure, structure-to-name, foreign language translation
- MolProp TK Molecular property calculation and filtering
- Quacpac TK Tautomer enumeration and charge assignment
- OEMedChem TK Matched molecular pair analysis, fragmentation utilities, and molecular complexity metrics
- Saiph TK: Extracting, transforming, and loading (ETL) files and records
References
- A New Atom-Additive Method for Calculating Partition Coefficients Wang, R., Ying, F., Lai, L.J., Chem. Info. Comput. Sci., 1997, 37, 615.
- Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties Ertl, P., Rohde, B., Selzer, P., J. Med. Chem., 2000,37, 3714.
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings Lipinski, C., et. al., Adv. Drug. Deliv. Rev., 1997, 23, 3.
- Prediction of drug absorption using multivariate statistics Egan, W.J., Merz, K.M., Baldwin, J.J., J. Med. Chem., 2000, 43, 3867.
- Molecular properties that influence the oral bioavailability of drug candidates Veber, D.F., Johnson, S.R., Cheng, H.Y., Smith, B.R., Ward, K.W., Kipple, K.D., J. Med. Chem., 2002, 45, 2615.
- A bioavailability score Martin, Y.C.,J. Med. Chem.,2005,48, 3164.
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch
Science Brief: Binding Free Energy Redefined: Accurate, Fast, Affordable
CUP XXV - Santa Fe March 10-12, 2026
Webinar: Novel Hits from Beyond the Known: ROCS X
Resources
Glimpse the Future through News, Events, Webinars and more
News
ROCS X: AI-Enabled Molecular Search Unlocks Trillions
Webinar
Webinar: OpenEye's Free energy prediction for drug discovery: Ideas at breakfast, discoveries by lunch


